In recent news, a weight loss drug, Ozempic, has led to severe health issues for some users, including a woman who now faces lifelong challenges due to a “life-threatening bowel injury” caused by the medication.
This case is part of a broader issue, with dozens of lawsuits filed against Novo Nordisk, the manufacturer of Ozempic and Wegovy, as patients experienced extreme side effects without prior warning. These legal actions highlight concerns over the safety and communication of potential risks associated with these drugs.
Legal Actions Against Drug Manufacturers

The New York Post reports that the pharmaceutical companies Novo Nordisk and Eli Lilly are currently facing legal scrutiny as they are accused of failing to adequately warn patients about the risk of gastroparesis, a condition resulting in stomach paralysis, from their drugs Ozempic, Wegovy, and Mounjaro.
With “thousands” more lawsuits expected to be filed, the situation brings to light the critical issue of patient safety and informed consent in medication use.
Legal Challenges Mount Against Eli Lilly

Eli Lilly faces over ten lawsuits related to Mounjaro, a diabetes medication also used for weight loss, similar to Ozempic and Wegovy.
Accusations center on inadequate risk warnings, particularly about gastroparesis—a condition causing severe vomiting and, in one distressing case, the loss of a patient’s teeth due to the severity of symptoms, according to The Daily Mail.
Understanding Gastroparesis and Its Impact
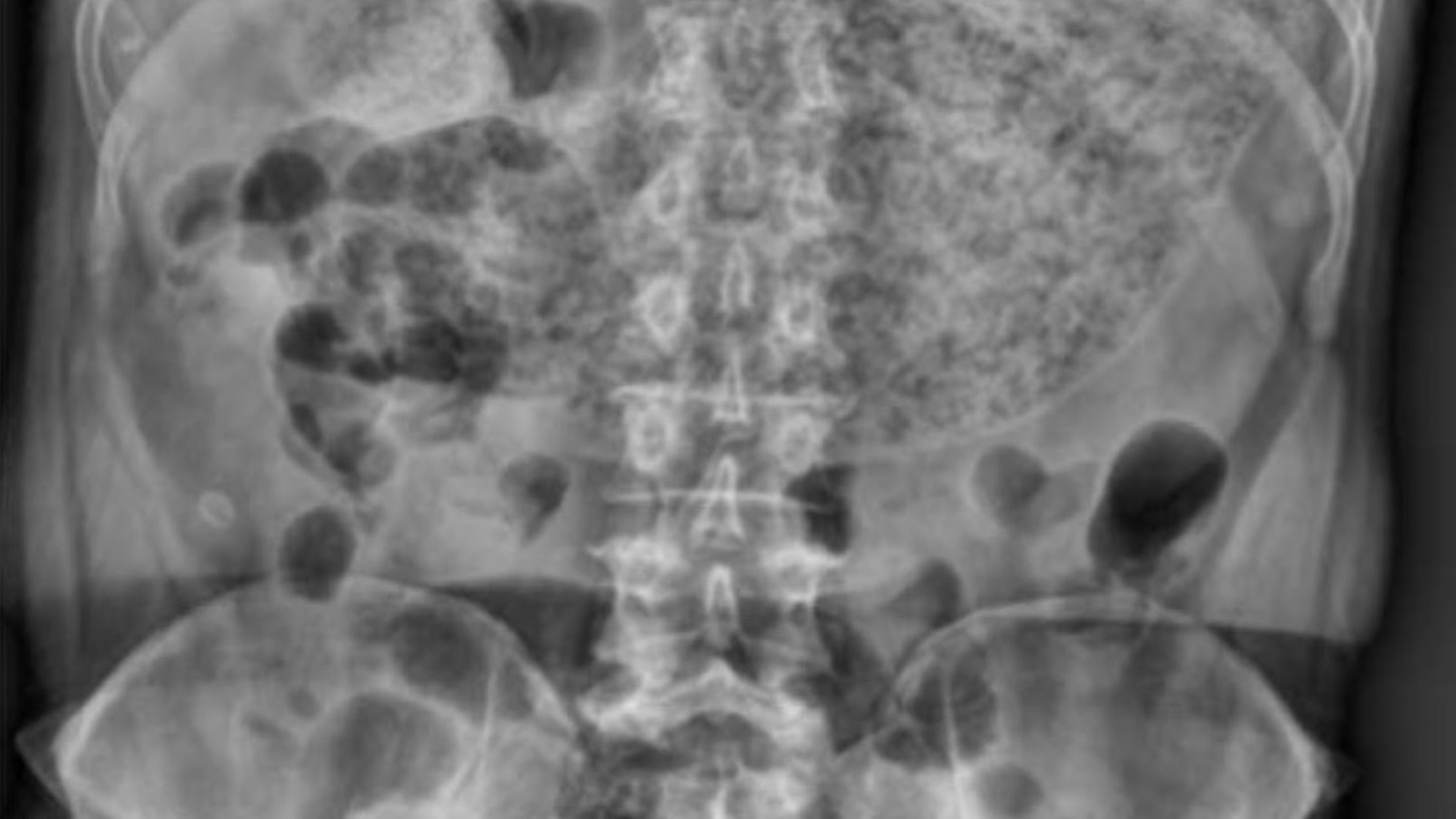
Gastroparesis, is a condition that can have life-threatening implications, leading to symptoms such as nausea, vomiting, and severe pain due to the buildup of food in the gut, according to the National Institute of Diabetes and Digestive and Kidney Diseases.
Patients who used Ozempic and Wegovy have reported suffering from this condition, which has significantly impacted their quality of life and, in some cases, resulted in permanent health issues.
The Scale of the Problem

The legal challenges against Novo Nordisk and Eli Lilly are growing, with more than 40 cases filed in federal courts across America, The Daily Mail reveals.
These lawsuits accuse the companies of not properly warning users about the risks of gastroparesis on the drugs’ packaging, a failure that has led to serious health consequences for numerous individuals.
Personal Accounts of Suffering

Victims of the side effects of these weight loss drugs have shared their distressing experiences, including undergoing extensive hospital stays and facing ongoing health issues.
For example, Brea Hand, a 23-year-old mother, told The Daily Mail, she suffered from gastroparesis and diabetic ketoacidosis after using Ozempic, leading to an intensive care admission where she was told she was close to death.
The Terrifying Ordeal

Hand revealed in her lawsuit, “They said my body was so acidotic that if I would have waited one more day that I wouldn’t have made it through.”
“It was scary. It was painful. I have not ever experienced that kind of pain in my entire life and I do not ever want to go through that again.”
Severe Consequences from Weight Loss Drugs
The Daily Mail also shares how another woman’s use of Ozempic led to a “life-threatening bowel injury,” requiring nearly nine hours of surgery.
Post-operation, doctors delivered a grim prognosis: she would endure pain indefinitely and could never expect to have a normal bowel movement again, highlighting the severe potential side effects of these widely used drugs.
The Legal and Regulatory Landscape

The lawsuits against the makers of Ozempic and Wegovy not only question the safety of these drugs but also examine the regulatory oversight provided by agencies like the FDA, The New York Post notes.
As these cases unfold, they raise important questions about the approval processes for medications and the adequacy of warnings provided to patients and healthcare providers.
The Role of Marketing in Medication Use
Novo Nordisk and Eli Lilly’s marketing strategies for their weight loss and diabetes drugs have come under scrutiny.
The New York Post reports that critics argue that the aggressive promotion of these medications may have overshadowed the potential risks, leading to a situation where patients were not fully informed about the possible adverse effects of the drugs.
The Wider Implications for Patient Safety

The ongoing lawsuits and reported cases of severe side effects from Ozempic and similar drugs are part of a broader concern about patient safety in the pharmaceutical industry.
These incidents highlight the need for more transparent communication about the risks associated with medications and for patients to be fully informed before starting any new treatment, via The Daily Mail.
Calls for Increased Transparency and Accountability
According to The New York Post, in response to the growing number of lawsuits and patient reports of adverse effects, there have been calls for increased transparency from pharmaceutical companies and for stricter regulatory oversight to ensure that patients are adequately warned about the potential risks of medications.
This situation serves as a reminder of the importance of patient safety and the need for comprehensive risk communication.








































