Haleon has issued a nationwide recall for eight lots of their popular Robitussin cough syrup.
The recall is due to “microbial contamination”, as reported by NBC News.
Specific Products Affected
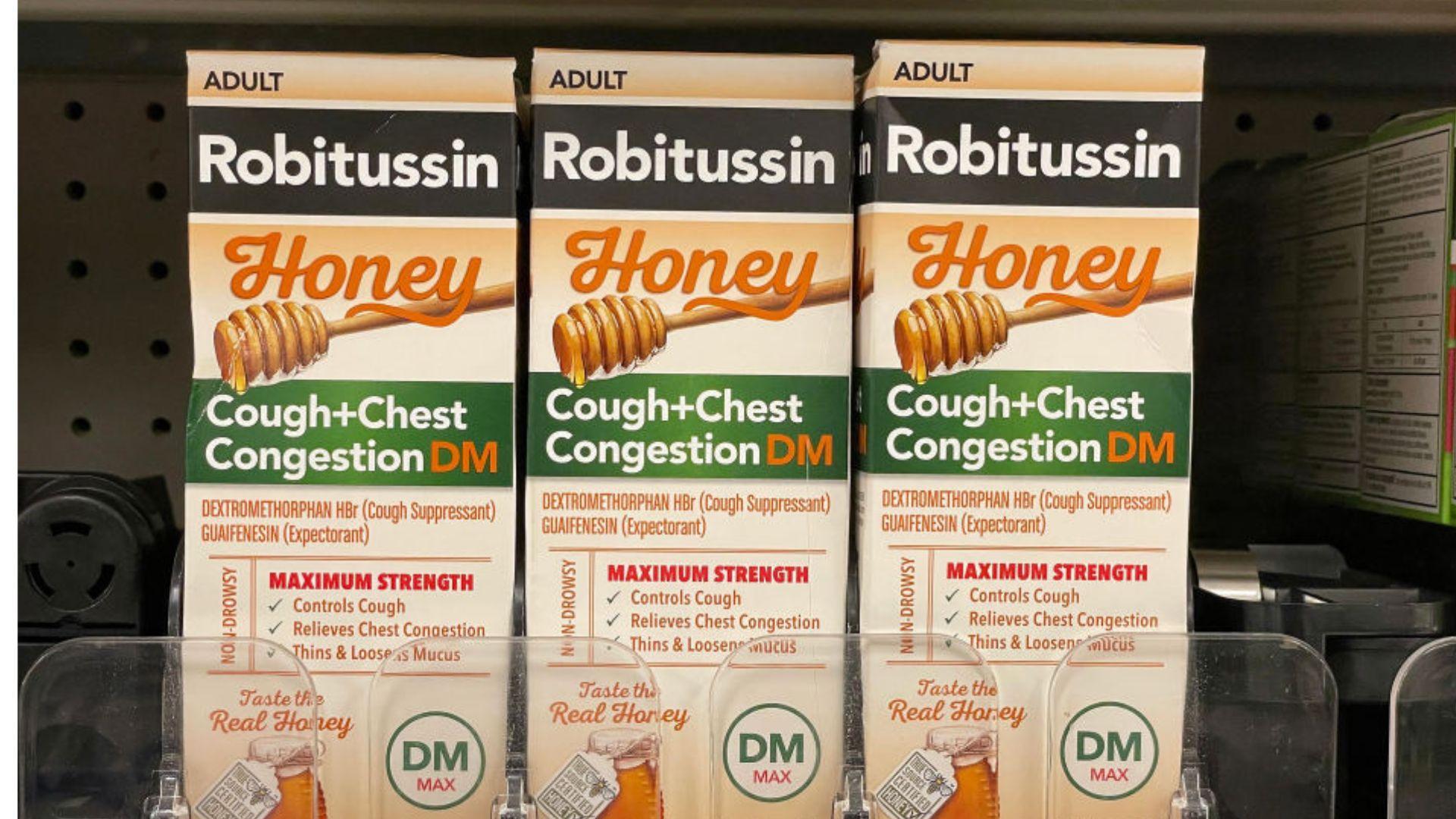
The New York Post reports that the specific products involved in the recall are Robitussin Honey CF Max Day and Nighttime.
These products are commonly used by many to relieve symptoms associated with respiratory issues.
Risk to Immunocompromised Individuals

According to the FDA’s public service announcement, the use of these contaminated products could result in “severe or life-threatening adverse events” for individuals with compromised immune systems.
This highlights the potential severity of the contamination and the risks it poses to a vulnerable segment of the population.
Potential Complications From Contamination
NBC News reports that the FDA’s warning includes the possibility of developing fungemia — the presence of fungus or yeasts in blood — and disseminated fungal infection.
These conditions can be potentially fatal, especially for those who are immunocompromised.
Risks for Non-Immunocompromised Individuals

The New York Post reports that individuals who are not immunocompromised should still avoid the recalled products.
They explain that the FDA advises that while non-immunocompromised people are unlikely to experience severe adverse effects, “the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.”
Haleon’s Advisory to Consumers

NBC News reveals that in response to the contamination, Haleon has urged customers who have purchased the affected product to stop its consumption immediately.
This direct appeal to consumers is part of Haleon’s effort to prevent any health issues that might arise from using the contaminated cough syrup.
Guidance for Those Who Have Used the Product

Haleon also advises individuals who have used the product to “contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product”, as per information from The New York Post.
Alerting a doctor is crucial for monitoring potential health effects that may have arisen from the use of the contaminated product.
Reporting Adverse Reactions

The FDA encourages consumers to report any adverse reactions or issues related to the product through the FDA’s MedWatch Adverse Event Reporting program.
This can be done online, or via regular mail, The New York Post reports.
No Reports of Symptoms Yet
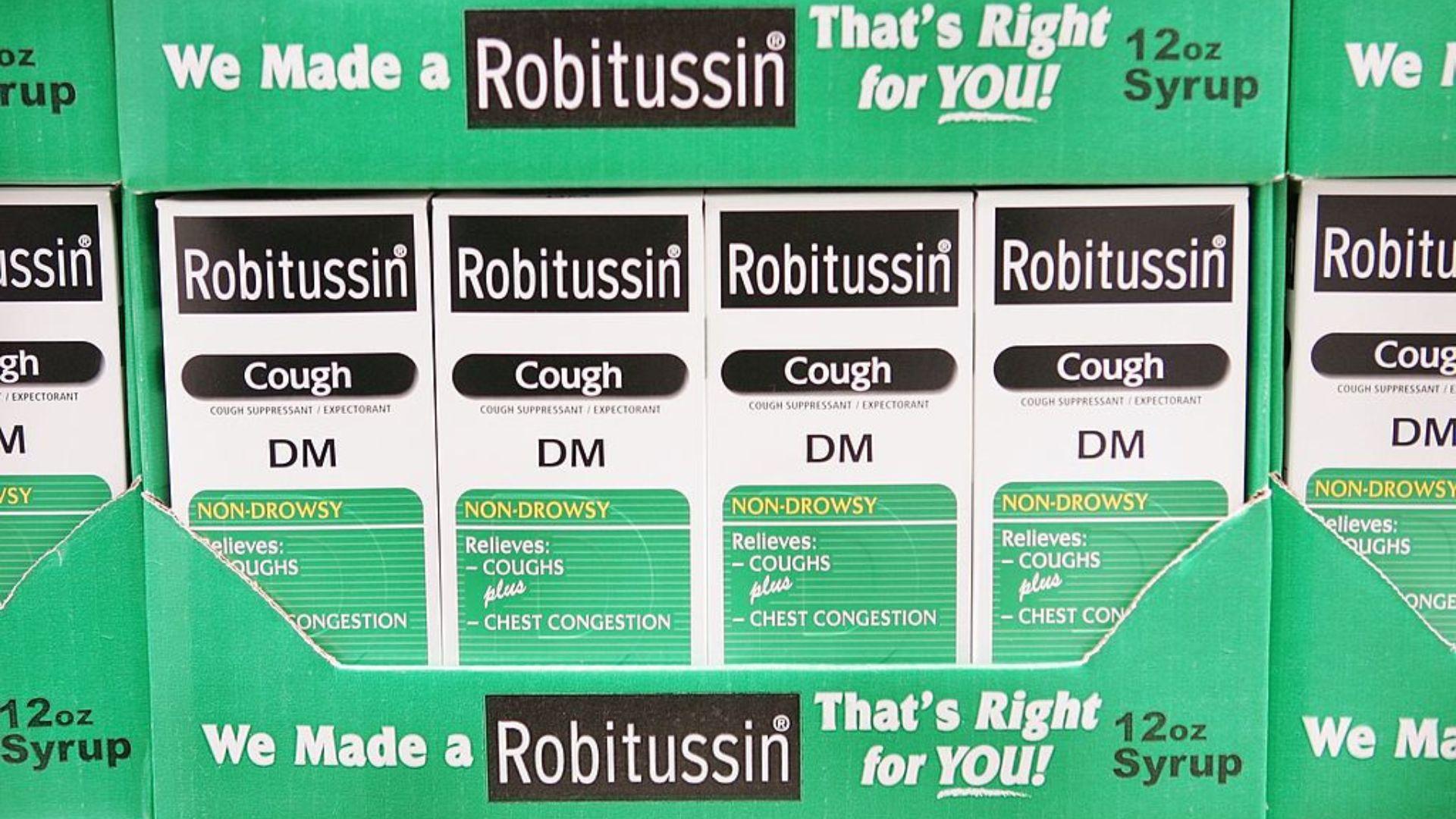
As of the time of the recall, Haleon has not received any reports of symptoms stemming from this issue, as reported by The New York Post.
This information, provided by Haleon, offers some reassurance that the recall is a precautionary measure and that no adverse effects have been reported so far.
Nationwide Scope of the Recall
The New York Post reports that the recall affects a wide range of consumers, given the nationwide distribution of Robitussin Honey CF Max syrups.
This illustrates the importance of widespread awareness and compliance with the recall instructions.
Consumer Action and Product Returns

The FDA reveals that in light of the recall, Haleon is actively notifying its distributors and customers to return the recalled products.
Haleon’s proactive approach ensures that the affected cough syrups are promptly removed from circulation, reducing the risk of any potential health issues.
FDA’s Role in Recall Process

The recall process of these eight lots of Robitussin is being conducted under the close oversight of the U.S. Food and Drug Administration.
The involvement of the FDA provides an additional layer of scrutiny and assurance, ensuring that the recall process adheres to strict health and safety guidelines.
Overview of Drug Recalls in Early 2024

Since January, the United States has seen at least four drug recalls, including the incidents involving lubricant eye drops, various IV bags, and Zenzedi or dextroamphetamine sulfate tablets, The Guardian reports.
These recalls have been prompted by a range of issues, including device and drug safety concerns, the potential for super-potent drugs, and mislabeled packaging.
A Nationwide Recall on ADHD Medication

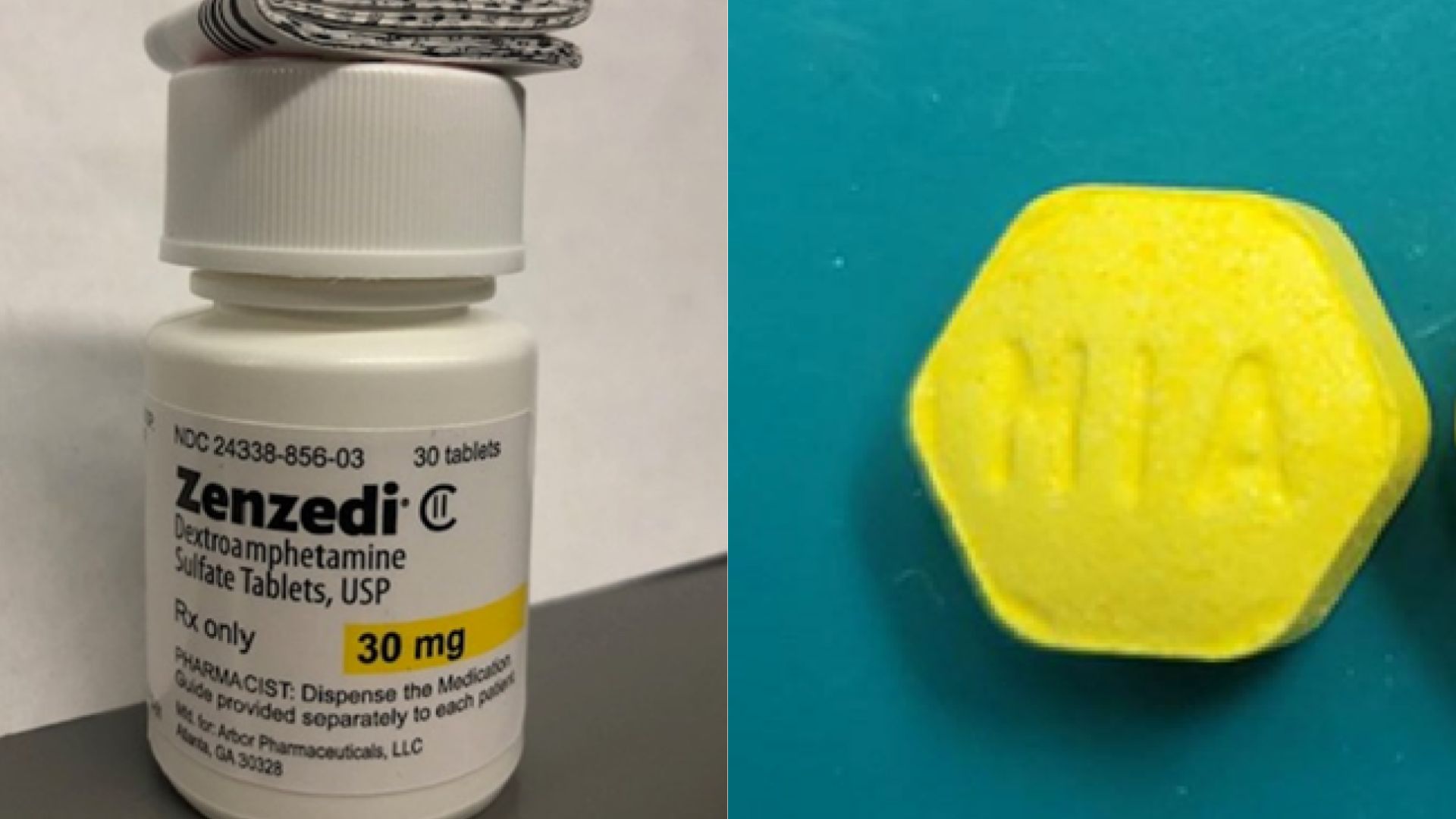
Azurity Pharmaceuticals initiated a recall of its ADHD and narcolepsy medication, Zenzedi, after a pharmacist discovered incorrect pills within a package of the drug.
The recall notice specifically targets one lot of Zenzedi 30 milligram tablets. This action was taken in response to finding tablets of carbinoxamine maleate, an antihistamine, in a bottle labeled as Zenzedi, according to the FDA.
The Risks of Medication Mix-Up

CNN reports that the active compound in Zenzedi is dextroamphetamine sulfate, a stimulant used for treating ADHD and narcolepsy.
The mistakenly included carbinoxamine maleate is a sedative, which poses significant health risks due to its opposite effect. The recall notice warns that taking carbinoxamine instead of Zenzedi may lead to drowsiness, increased eye pressure, urinary obstruction, and thyroid disorder.
Complications Amidst ADHD Drug Shortage
This recall comes at a particularly challenging time, amidst a national shortage of ADHD drugs that began in October 2022, CNN notes.
The shortage has already made it difficult for individuals with ADHD to find consistent medication. The recalled lot is identified by the lot number F230169A, with an expiration date of June 2025.
Guidance for Consumers with Recalled Medication

According to CNN, consumers in possession of the recalled medication are advised to return it to their pharmacy immediately.
Those experiencing any adverse effects from the drug are encouraged to contact their health care provider. Azurity has reported no serious injuries related to the medication swap, but the situation underscores the importance of vigilance among patients and pharmacists alike.
FDA Alert on Unapproved Eye Drops

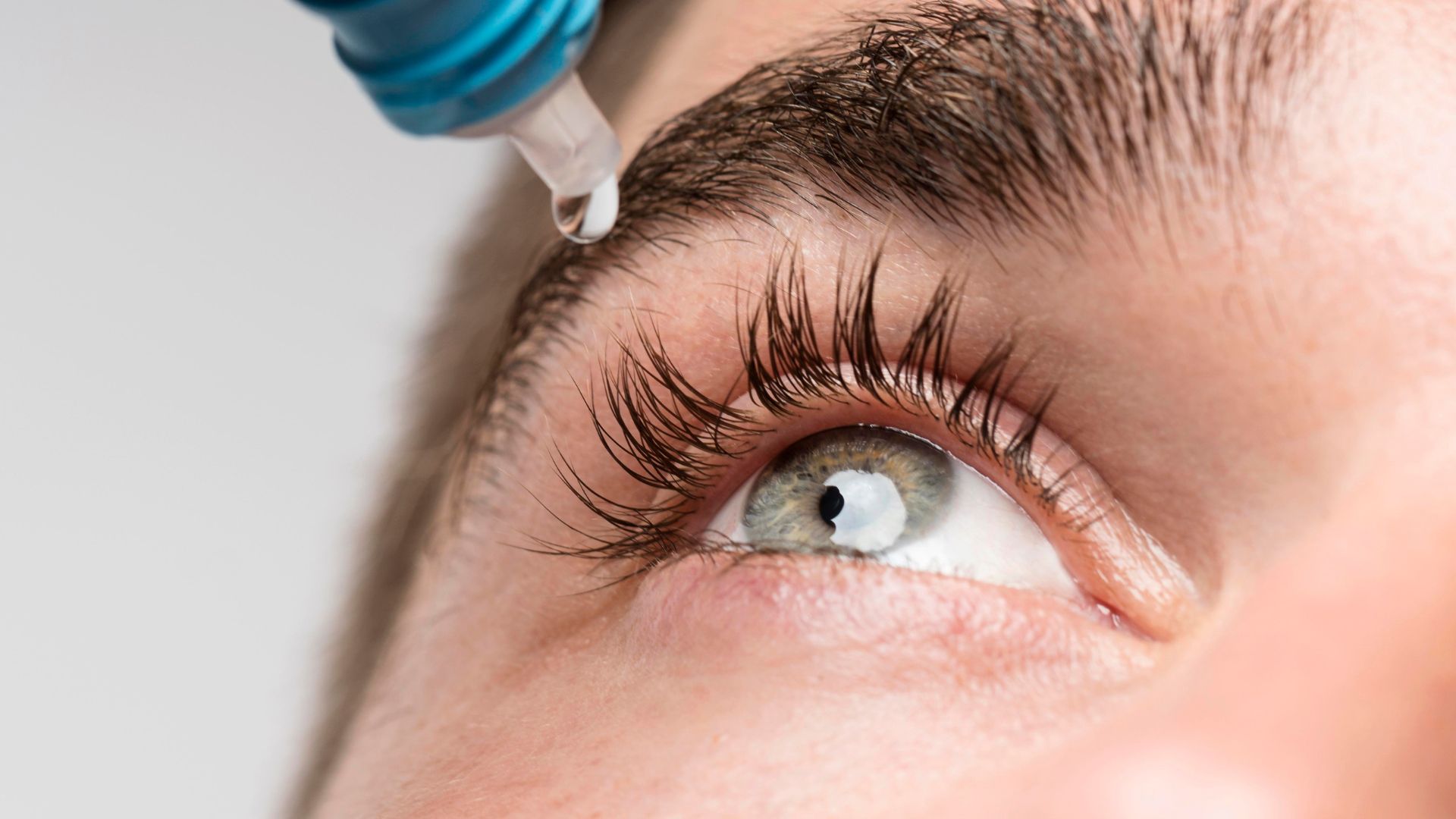
The FDA has also issued a warning to consumers about the dangers of purchasing or using South Moon, Rebright, or FivFivGo eye drops.
These products are unapproved and pose a risk of eye infection. They mimic the appearance of Bausch + Lomb’s Lumify eye drops but lack the authorized active ingredient for redness relief.
The Contamination Concern with Copycat Eye Drops

FDA testing revealed that South Moon eye drops were contaminated with Burkholderia cepacia complex, a bacteria group capable of causing antibiotic-resistant infections.
Although Rebright eye drops tested negative for contamination, the FDA still advises against their use. These findings highlight the critical issue of product safety and the risks associated with unapproved drugs.
The Origins and Availability of Counterfeit Eye Drops

Investigations into the source of these counterfeit eye drops are ongoing. South Moon is labeled as being produced by Shantou Cross-border Premium Products E-Commerce Co. Ltd. in China.
The FDA continues to probe into the matter, emphasizing the importance of purchasing eye care products from reputable retailers to avoid such risks.
FDA’s Advisory Against Fake Eye Care Products

While no adverse events specifically naming South Moon, Rebright, or FivFivGo products have been reported to the FDA, the agency has received complaints related to potentially fake Lumify products.
These complaints include product quality concerns, eye irritation, pain, and infection, underscoring the need for caution when selecting eye care products.
Identifying Authentic Lumify Products

Consumers are advised to be vigilant in distinguishing between authentic Lumify eye drops and their counterfeit counterparts.
Some of the fake products may falsely include “Bausch + Lomb” branding, misleading consumers. Authenticity checks and purchasing from licensed pharmacies are recommended to ensure product safety and efficacy.
Reporting Adverse Events to the FDA
Health care professionals and consumers are encouraged to report any adverse events or side effects related to the use of these counterfeit eye products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
This reporting mechanism is vital for monitoring product safety and preventing further incidents.
The Importance of Drug Safety Awareness

The recent recalls and safety warnings emphasize the importance of drug safety and the vigilance required by consumers, health care providers, and regulatory bodies.
Ensuring the integrity of medications and health care products is crucial for patient safety, requiring ongoing attention and responsiveness to potential risks and contamination.








































