Electrical stimulation devices (ESDs) are controversial tools used to prevent self-harm or aggressive behavior. They work by delivering shocks to the skin of individuals, primarily used at the Judge Rotenberg Educational Center.
The center says these devices are a last resort for severe cases, but the ethical debate surrounding their use is far from black and white. Critics argue the method is inhumane, sparking a nationwide conversation on the rights and treatments of individuals with developmental disorders.
A History of Controversy

The debate over ESDs isn’t new. In 2013, a United Nations report declared the Rotenberg Center’s use of shock devices a form of torture.
This international condemnation brought global attention to the practice, igniting fierce debates on human rights, ethical treatment, and the dignity of individuals with developmental challenges. Despite the controversy, the center continued using ESDs.
FDA’s First Attempt to Ban

In 2020, the FDA attempted to ban ESDs, stating a better understanding of the dangers they present to public health (via Fierce Biotech). However, their efforts hit a roadblock when a federal court ruled that the agency lacked the necessary authority.
This decision marked a significant setback in the movement against ESDs but also set the stage for a broader legislative review and public discourse.
Rising Public Concern

As awareness grows, so does public concern. According to the FDA, the devices pose numerous psychological risks, including depression, anxiety, and PTSD, not to mention physical risks like pain and burns.
Critics argue that many individuals subjected to ESDs have disabilities that impede their ability to communicate or make informed treatment decisions, raising significant ethical questions.
A Change in Legislation

In response to the court’s ruling and growing public pressure, Congress revised the law, clarifying the FDA’s authority to regulate ESDs.
This legal clarification has paved the way for the FDA to take a second stab at banning these controversial devices. The move represents a critical step toward addressing the complex interplay between medical ethics, patient safety, and regulatory oversight.
Understanding the Risks

The FDA has outlined significant risks associated with ESDs: “These devices present a number of psychological risks, including depression, anxiety, worsening of underlying symptoms, development of post-traumatic stress disorder, and physical risks such as pain, burns, and tissue damage.”
This stark warning underlines the urgency of reevaluating their use in behavioral management.
The FDA Strikes Back

With new legislative backing, the FDA announced a renewed effort to ban ESDs, citing advancements in medical treatments that render the harsh devices unnecessary (via CNN).
This move has been welcomed by many as a long-overdue step toward more humane treatment options for individuals with developmental and intellectual disabilities.
Alternatives to Shock

Advancements in medical and behavioral sciences, offering safer, more effective treatments, support the shift away from ESDs.
These alternatives, which include therapy, medication, and non-invasive behavioral interventions, can reduce or stop self-injurious or aggressive behavior without the substantial risks posed by ESDs.
Public Opinion and Action

The FDA’s proposal is open for public comment until May 28, allowing citizens to voice their opinions.
This period of public engagement is crucial for shaping the final decision and underscores the democratic process in regulatory decisions impacting vulnerable populations.
A Glimpse into the Judge Rotenberg Center

According to U.S. News & World Report, the Judge Rotenberg Center remains the sole user of ESDs in the U.S. Some students at the center, court-approved for the treatment, wear devices that can administer shocks through a backpack system.
This practice has faced intense scrutiny and symbolizes the broader ethical challenges in treating behavioral and developmental disorders.
Personal Stories and Repercussions
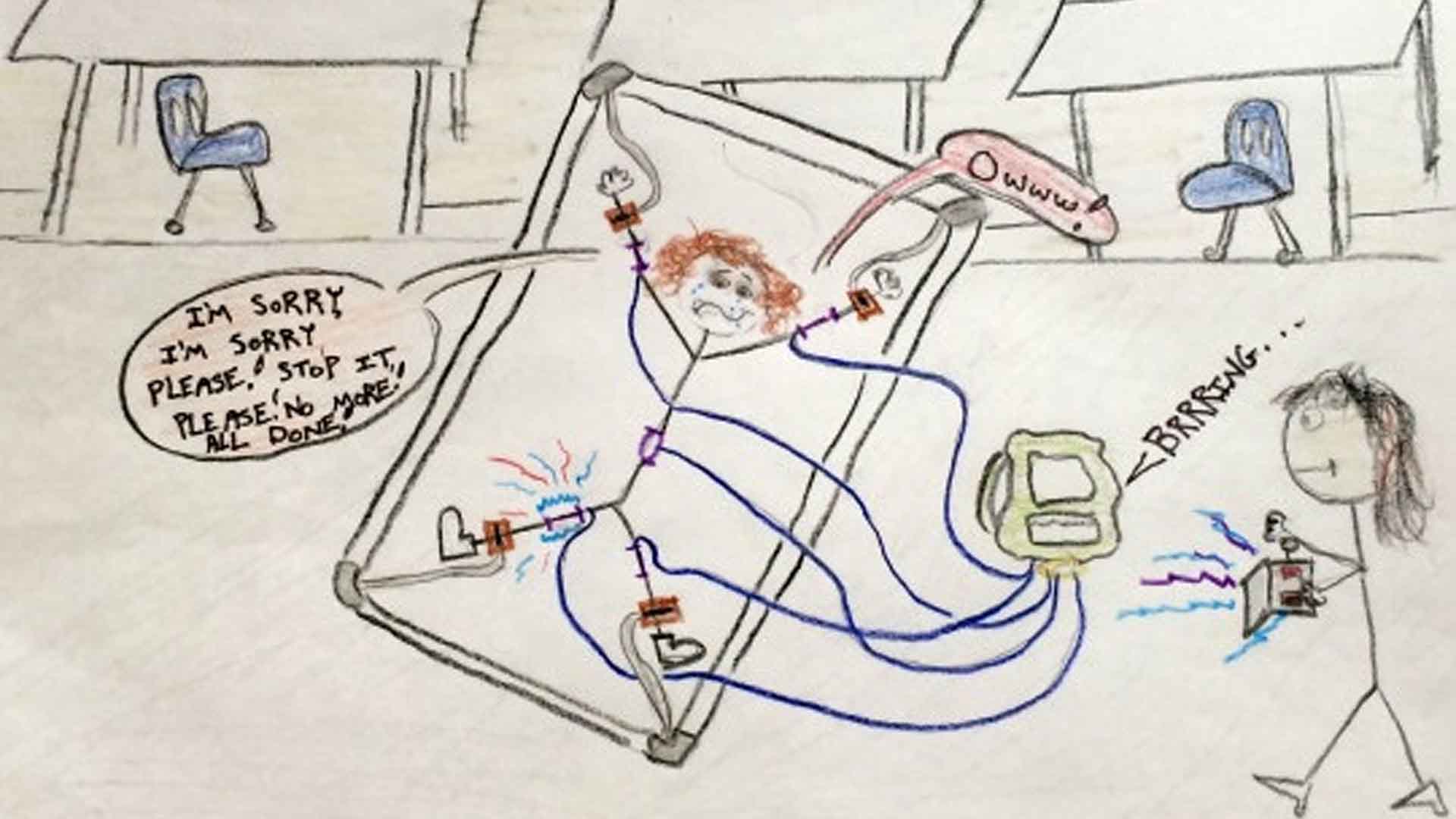
Behind the statistics are real stories of individuals and families affected by ESDs. These personal accounts highlight the human cost of the devices, shedding light on the lasting physical and emotional scars left by such treatments.
Their stories are a powerful reminder of the stakes involved in the ongoing debate over ESD use.
Looking Ahead

As the FDA considers the future of ESDs, the needs of those currently undergoing such treatment will be a significant concern.
The proposed rule underscores a commitment to a safer, more ethical approach to behavioral management, signaling a potential end to a long-standing and controversial practice.








































